Bond Angle of Cyclohexane
CCC bond angles in cyclohexane chair conformation are very close to the canonical tetrahedral angle of 10947. What are the bond angles in cyclohexane.

Convert Newman Projection Of Cyclohexane To Bond Line Chemistry Textbook Chemistry Lessons Study Chemistry
In the chair form of cyclohexane the carbon atoms and the bonds around them are almost perfectly tetrahedral.

. The molecular formula of cyclohexane is C6H12. A stable conformation adopted by cyclohexane. If cyclohexane has a planar structure then the bond angles would be 120.
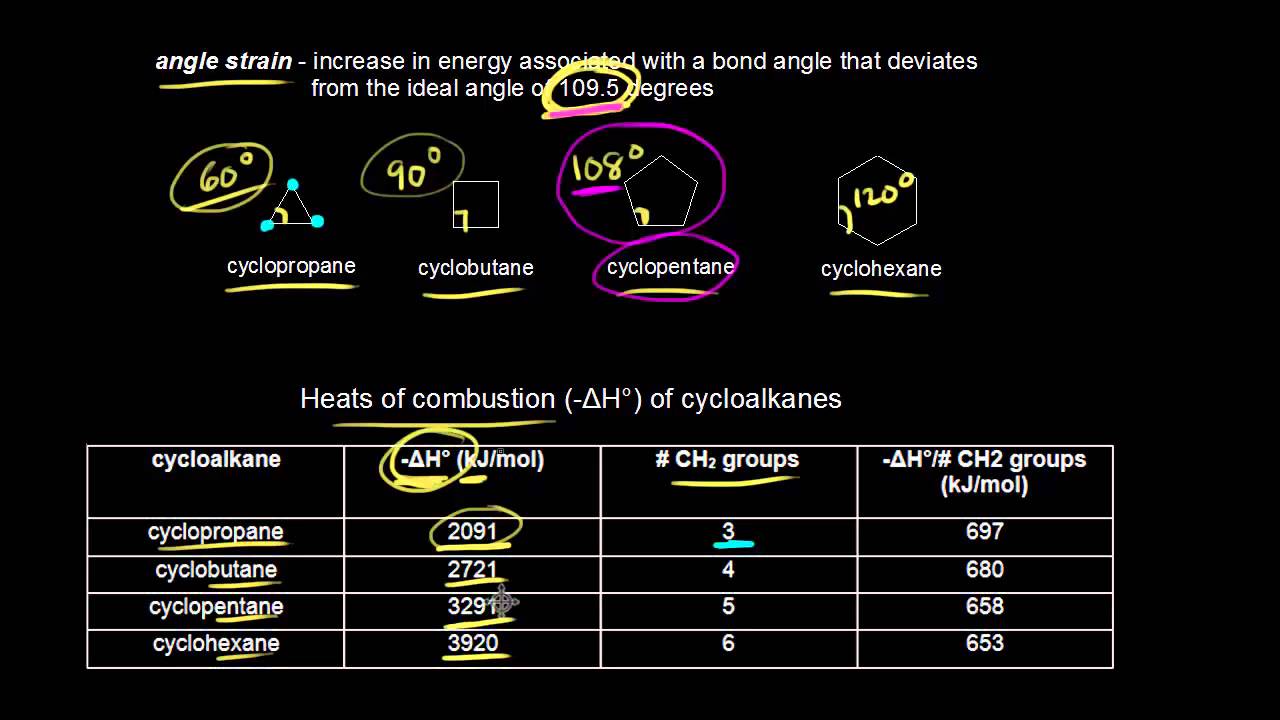
The crystal structure shows a. The same is true for angle HCH and the four angles CCH. All of the carbon atoms in cyclopropane are tetrahedral and would prefer to have a bond angle of 1095 o The angles in an equilateral triangle are actually 60 o about half as large.
For cyclohexane C-atoms tend to form. The hydrogens on adjacent carbons are also arranged in a perfect staggered. This deviation in bond angle from the ideal.
If cyclohexane has a planar structure then the bond angles would be 120. Cyclohexane C 6 H 12 Cyclopropane C 3 H 6. Conformation because there is steric hindrance among the flagstaff H-atoms and all the C H bonds are eclipsed.
A cyclohexane conformation is any of several three-dimensional shapes that a cyclohexane molecule can assume while maintaining the integrity of its chemical bonds. The 60º bond angles are much smaller than the optimum 1095º angles of a normal tetrahedral carbon atom and the resulting angle strain dramatically influences the chemical behavior of. This angle strain often enhances the chemical reactivity of such compounds leading to ring cleavage products.
The Carbon and Hydroxide bond angle is 1045 forming a bent shape. In planner six member hexagonal shape it has bond angles of 120 0 and so it must have angle strain because all angle are greater than 1095 0. In the chair conformation of cyclohexane all the carbons are at 1095º bond angles so no angle strain applies.
This deviation in bond angle from the ideal bond angle 1095. The bond angles would necessarily be 120º 105º larger than the ideal tetrahedral angle. So the C-C-H angles will be almost exactly 1095 degrees.
The molecular formula of cyclohexane is C6H12. A planar structure for cyclohexane is clearly improbable. For a cyclopentane if it is a regular planar pentagon then its bond angles would be 108 but if it is in tetrahedral shape 1095 would be the bond angle.
Its stability results from the elimination of angle strain all bond angles are 1095 and torsional strain all groups on adjacent carbon atoms. What are the bond angles in cyclohexane. This bond angle keeps the carbon atoms as close as possible without them interfering with each other.
But if cyclohexane were in a flat hexagon the bond angle would be. This bond angle keeps the carbon atoms as close as possible without them. Cyclohexane Conformation Carbon atoms like to form bond angles of 1095 degrees.
Stability Of Cycloalkanes Organic Chemistry Books Organic Chemistry Chemistry

Cyclohexane Conformations Master Organic Chemistry Organic Chemistry Chemistry Chemistry Labs

Cyclohexane Chair Flip Summary Of How To Draw A Ring Flip Mcat Study Chemistry Student Learning

Cyclohexane Conformations Master Organic Chemistry Organic Chemistry Teaching Chemistry Chemistry Lessons
No comments for "Bond Angle of Cyclohexane"
Post a Comment